45 medication labels must include
Prescriptions- Label Flashcards - Quizlet Here is a list of all the things that need to go on a prescription label for a non-control: 1. name of pharmacy 2. address of pharmacy 3. telephone number of pharmacy 4. number of the prescription 5. date the prescription is filled 6. name of patient**** 7. name of prescriber 8. initials of the pharmacist dispensing the Rx Prescription Product Labeling - University of North ... Labels for controlled substances in schedule II, III, and IV must also contain the statement "Caution: Federal law prohibits the transfer of this drug to any person other than the patient for whom it was prescribed." in the form of an auxiliary label. Recommended Label Infromation
How Do I Use Prescription Drug Labeling | FDA Types of FDA-approved patient labeling include Patient Package Inserts (PPIs), Medication Guides (MGs), and Instructions for Use (IFUs). A Medication Guide must be provided to the patient whenever...

Medication labels must include
Pharmacology Chapter 5 (Prescriptions and Labels) - Quizlet the FDA specifies exactly what must appear on the drug label. which include:-foods to avoid-drugs to avoid-activities to be avoided while taking the drug-clear instructions to health-care practitioners-and drug ingredients. a medication label contains what information: Drug labeling, Information about Drug labeling - FAQs Each product must contain a label with "Supplement Facts" in bold letters onthe front panel. This is the manufacturer's opportunity to identify the product. Below "Supplement Facts," the panel must state the serving size. This isdetermined by the manufacturer with no input from the FDA. Pharmacist FAQs - NCBOP A. The following information must be on every prescription label: 1. Name and address of the dispensing pharmacy. 2. Serial number of the prescription. 3. Date of the prescription. 4. Name of the prescriber. 5. Name of the patient. 6. Name and strength of the drug. 7.
Medication labels must include. OTC Labeling Requirements - FindLaw Below the "Drug Facts" section, a "Uses" section will list the approved or monograph indications for the drug. The next section of the label is "Warnings." In addition to specifying where warnings must appear, FDA has also prioritized the order in which warnings are presented. The first warning, if applicable, is: "For external use only." What's on a prescription label? - Knowledge is the best ... In general your label will contain the following information:. Parts of a Prescription Label Rollover A-K below to see the various part of a prescription label. * A Drug Identification Number (DIN) is an eight digit number assigned by Health Canada to a drug product prior to being marketed in Canada. Patient Labeling 101 - Food and Drug Administration Patient labeling should be written at a 6 to 8 th grade reading level z Use of certain fonts: Verdana, Arial, or APHont size 11 or greater for better visibility z Use of text boxes, bold font, and... How to Label Prescription Medication for Veterinary ... A label should include the following components: The name of the veterinary practice, its address, and contact information The veterinarian's name, the patient's name and species, and the client's...
Chapter 5: Prescriptions and Labels Flashcards | Quizlet Drug Labels Regulated by the Food and Drug Administration (FDA), which determines what needs to be on the label Dispensing pharmacist's label must include: Pharmacy name, address, and phone number Dispensing date Dispensing date may differ from the date on the prescription. Rx number, which identifies this unique prescription in the computer system 4. Documenting Medications (MAR). | Aplmed Academy NOTE: Medication Labels cannot be altered, they must be re-written. Directions on medication labels from pharmacy are checked against the MAR. If there is a discrepancy between the information on the MAR and the medication label, check the order in the resident's record. (The label on medication cannot be changed by anyone. FDA Says Drug Labels Must Include Clear Guidance for ... FDA Says Drug Labels Must Include Clear Guidance for Pregnant Women Written by Stacey Feintuch — Updated on December 5, 2014 Revamped drug labels will give pregnant and breastfeeding women more ... How to Read Over-the-Counter and Prescription Drug Labels Some labels include a seventh section with a phone number to call if you have questions or comments. The Drug Facts label for the over-the-counter drug acetaminophen, known by the brand name Tylenol, includes information about ingredients, uses, warnings and directions. Active Ingredient and Purpose.
What Is a Drug Label? | The Motley Fool Drug labels include instructions, ingredients, and a lot more information. Here's what you need to know from a healthcare investor's standpoint. Medicines: packaging, labelling and patient ... - GOV.UK Labels must include warnings for safe use of the medicine. All products that contain paracetamol must include statutory warnings. Additional warning statements must be included on the packaging of... PDF Labeling on the Sterile Field: Improve Patient Safety and ... Labeling must include: Name of medication or solution, strength, date, and time Label one item at a time. Single items must also be labeled. Reading Medication Labels - Basicmedical Key Reading Medication Labels Objectives After reviewing this chapter, you should be able to identify: 1. The trade and generic names of medications 2. The dosage strength of medications 3. The form in which a medication is supplied 4. The total volume of a medication container where indicated 5.
FAQ: What information is required to be displayed on the ... Labels must include warnings about the safe use of the drug. All products containing paracetamol must contain mandatory warnings. Additional warnings must be included on the packaging of certain medications.
Chapter 8 NHA237 Flashcards - Quizlet If nothing is indicated on the prescription for refills, then the technician should: Check with the prescriber to see how many refills he or she wants. Tell the pharmacist. Enter the abbreviation, prn, (Latin, pro re nata) meaning as needed for refills. Enter zero refills. Enter zero refills.
Pharmacist FAQs - NCBOP The following information must be on every prescription label: 1. Name and address of the dispensing pharmacy. 2. Serial number of the prescription. 3. Date of the prescription. 4. Name of the prescriber. 5. Name of the patient. 6. Name and strength of the drug. 7.
Safe Labeling Helps Prevent OR Medication Errors - OR Today Label information must include a medication's name and strength as well as amount when medications are mixed (as with antibiotic irrigations, tumescent and heparin solutions, and epinephrine). The unit of measure — percent, grams, milliliters, or units — must be recorded along with the date the medication is prepared.
5 Things to Look For in a Prescription Medication Label ... 5 Things to Look For in a Prescription Medication Label. According to a 2006 Institute of Medicine report, about 1.5 million preventable medication errors are made each year. Prescription drugs are a double-edge sword. They can treat and manage diseases, making it possible to lead a better life.
The Over-the-Counter Medicine Label: Take a Look | FDA All nonprescription, over-the-counter (OTC) medicine labels have detailed usage and warning information so consumers can properly choose and use the products. Below is an example of what the OTC...
Ch 8 Pharmacy Flashcards & Practice Test - Quizlet A legal prescription label must include all of the following except: A. Directions for use B. Date the prescription was dispensed C. Name, address, and telephone number of the prescriber D. Name, address, and telephone number of the dispensing pharmacy Name, address, and telephone number of the prescriber
A Guide To Veterinary Prescription Label Requirements ... A Sample Label For Veterinary Prescription Drugs. This sample label for veterinary prescription drugs shows a best practice example of the required fields, type sizes and warnings that are most effective. What Is Required On A Veterinary Prescription Label. As shown in the above example, the actual container must include the following information:
Prescription Drug Labeling Resources | FDA FDA-approved patient labeling [Medication Guides, Instructions for Use, and Patient Information (also called Patient Package Inserts)], and Carton and container labeling. The PI has two formats:...
How to read prescription drug labels - BeMedwise Navigating the "medicine label" of a prescription drug: How to read and understand the information in prescription labels Whenever you are prescribed a medication, you should read and follow the information in the medication's "label" in order to ensure your safety. All prescription medicine containers include information on the label including the patient's name, the […]
Pharmacist FAQs - NCBOP A. The following information must be on every prescription label: 1. Name and address of the dispensing pharmacy. 2. Serial number of the prescription. 3. Date of the prescription. 4. Name of the prescriber. 5. Name of the patient. 6. Name and strength of the drug. 7.
Drug labeling, Information about Drug labeling - FAQs Each product must contain a label with "Supplement Facts" in bold letters onthe front panel. This is the manufacturer's opportunity to identify the product. Below "Supplement Facts," the panel must state the serving size. This isdetermined by the manufacturer with no input from the FDA.
Pharmacology Chapter 5 (Prescriptions and Labels) - Quizlet the FDA specifies exactly what must appear on the drug label. which include:-foods to avoid-drugs to avoid-activities to be avoided while taking the drug-clear instructions to health-care practitioners-and drug ingredients. a medication label contains what information:



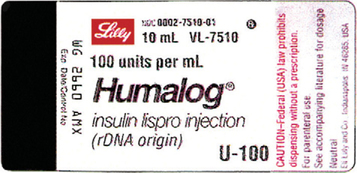







Post a Comment for "45 medication labels must include"